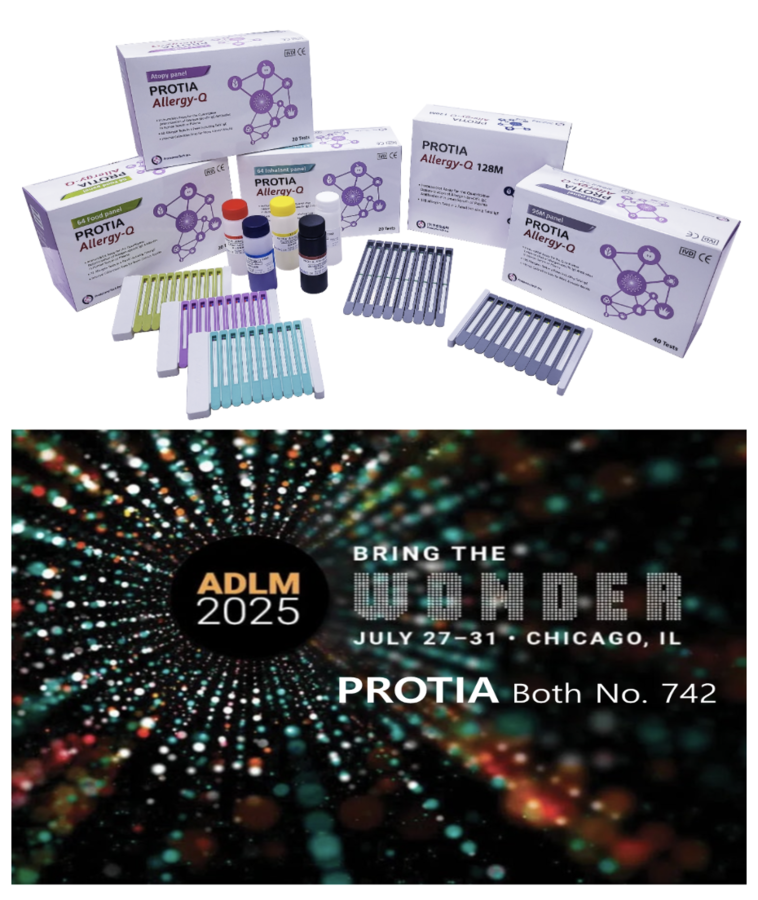
New Launch Highlight: Allergy-Q 192D Delivers Precision Across 176 Allergens in One Test
NEW YORK, NY, August 04, 2025 /24-7PressRelease/ — PROTIA, a leading in-vitro diagnostics company, announced its participation in ADLM 2025 (Association for Diagnostics & Laboratory Medicine, formerly AACC), the world’s largest clinical diagnostics exhibition, held from July 27 to 31 at McCormick Place in Chicago, USA.
At the exhibition, PROTIA showcased its flagship product—the multiplex allergy diagnostic kit—alongside a range of innovative diagnostic solutions featuring unique technological advantages, highlighting its strong competitiveness in the global market.
This participation marked the beginning of PROTIA’s strategic expansion into the Americas, positioning North, Central, and South America as key target markets. In response to the growing demand for allergy and immune diagnostics in major countries such as the U.S., Canada, Mexico, and Brazil, the company focused on strengthening its regional distribution networks and expanding partnerships.
Among the products featured was PROTIA’s newly launched multiplex allergy test kit, PROTIA Allergy-Q 192D. This high-performance in-vitro diagnostic product can precisely analyze up to 176 allergen responses—including foods, pollen, mold, drug allergens like penicillin shock, and recombinant protein allergens—with a single blood test. The product is highly regarded for its diagnostic accuracy and testing efficiency, and it provides valuable data for developing patient-specific treatment strategies. PROTIA also offers a wide range of customizable test options—40, 60, 100, 120, and 180 allergen panels—to meet diverse customer needs in allergy diagnostics.
In addition, the company introduced new diagnostic solutions with distinctive advantages, such as a multiplex test for 18 autoimmune diseases, a 90-item delayed food hypersensitivity test, and a next-generation antimicrobial susceptibility test that reduces turnaround time from 3 days to 1 while doubling the number of antibiotics tested (up to 40 types).
Through its participation in ADLM 2025, PROTIA further solidified its strategy to expand into the diagnostics market across the Americas, focusing on identifying local distribution partners, developing market-specific product strategies, and enhancing brand awareness.
A PROTIA representative stated, “Leveraging our accumulated technological expertise in allergy and immune diagnostics, we deliver innovative solutions tailored to customer needs. We will continue to strengthen our presence in the global in-vitro diagnostics market by addressing the diverse diagnostic demands of the Americas.”
—
For the original version of this press release, please visit 24-7PressRelease.com here
Legal Disclaimer:
The content on this page is syndicated from independent third-party providers. Kyrion Media makes no warranties or representations regarding the accuracy, completeness, legality, or reliability of the information, including text, images, videos, or licenses. If you are affiliated with this content or have any complaints, copyright concerns, or requests for removal, please contact us at retract@kyrionmedia.com with the specific URL of the content in question. We will review and address valid requests promptly.